AQUATIC KIT x2 FILTERS (MAXI)
For use in all countries
- All kit users must familiarize themselves with data requirements set out in the Sample Manifest, prior to sampling.
- Please ensure all information sent to NatureMetrics is in English.
- Datasheets must be completed using a pencil only.
- Kits should be used within one year.
USING THE KIT
Aquatic x2 filter eDNA Kits are designed to allow clients to filter more water and subsequently obtain more DNA for additional analyses.
Kit contents
1 x pair of nitrile gloves

1 x 5 L sampling bag 1 x specimen bag
2 x enclosed filters (0.8 μm pore size, polyethersulfone)
1 x 60 mL Luer Lock plastic syringe
1 x sampling datasheet
2 x resealable bags, each containing: 1 x small syringe filled with 1.5 mL of DNA preservation buffer (sealed with reusable Luer Lock cap) and 1 x separate Luer Lock cap
Vampire Pump

1 x pump head (A)
1 x drive unit (B)
YOU WILL NEED
- Device to capture GPS coordinates (lat / long) (e.g. GPS capable smartphone or standalone GPS device)
- Pencil (to complete the datasheet)
- Permanent marker (for labelling filters)
- Sterile ladles or new mineral water bottles (for collecting subsamples)
More than one person using the kit? Please purchase extra gloves.
To minimize waste we provide one pair of gloves per kit. If two or more people will be handling the contents of the kit and collecting an eDNA sample, you will need to purchase additional pairs of gloves – enough for one pair of gloves for each individual handling the kit. We recommend purchasing medical-grade nitrile gloves.
Sampling from a boat, or in waterbodies with inaccessible shorelines?
Sterile ladles to assist with sample collection can be purchased from NatureMetrics. If you need to acquire some, please contact us. In the absence of ladles, brand new empty mineral water bottles can be used.
MAXI Aquatic eDNA Kits come with a 5 L sampling bag and two filters. Up to 5 L of water should be passed through each filter, so two water samples may need to be collected in the same manner with the same sampling bag or sampling device (e.g. Kemmerer or Niskin sampler). Note that the actual volume passed through each filter may be less than 5 L due to turbidity of the sampled water (see Advice Note for recommended volumes in different environments). We provide one sampling bag as standard to minimize waste – you may want to source extra sterile sampling bags (NatureMetrics can recommend suppliers) for your sample collection if you do not want to reuse the sampling bag. A separate protocol for processing larger volumes of water using pump filtration is also available from NatureMetrics.
TIPS FOR GETTING THE BEST RESULTS
Avoiding Inconclusive Results:
To get the best results possible, to avoid inconclusive results, and to avoid situations where data cannot be reported from a sample, please read our HOW TO AVOID INCONCLUSIVE RESULTS guide before undertaking fieldwork.
In summary, when inconclusive results occur, it is because the target DNA was not detected, DNA was degraded, or PCR (a crucial part of the lab process) was inhibited.
Key steps you can take to help avoid inconclusive results: do not leave your samples exposed to sunlight or heat, and return samples to NatureMetrics as soon as possible after sampling.
Avoiding contamination:
To increase confidence in results, avoiding contamination of samples is highly important. Sealed kits are sterile until opened; do not open more than one kit bag at a time, keep kit contents inside the bag before use, and put on the provided gloves before touching other kit components. Whilst wearing gloves, avoid touching anything not necessary to sample collection to minimize introducing external DNA into the sample. Change gloves between each kit. If any reusable tools are used, these should be decontaminated, using a disinfectant wipe or a bleach solution, between each sample.
USER PROTOCOL
PART 1: COLLECTING THE WATER
Ideal sampling strategies will vary among habitats and monitoring contexts. We recommend that you speak to a member of our team to plan your survey. If planning your own survey, it is important to follow the guidance outlined in the Advice Note, as suitable sampling strategies differ for different waterbodies.
We also recommend the inclusion of field negative controls (blanks), as part of your sampling strategy, to detect possible contamination introduced during sampling. These are purified water (i.e. mineral, deionised, or MilliQ water) processed in the same way as water samples (see Advice Note for details). NatureMetrics provide a free kit to be used as a field blank – one for every 20 kits purchased.
Once a suitable sampling strategy has been decided and planned:
1. Open the kit bag. Kits should be used one at a time to minimize contamination between samples. Do not open more than one kit at a time, especially if kits are to be used at different sampling locations. Put on the gloves provided, and minimize contact with the water, to avoid introducing DNA (from yourself, soil, or other waterbodies) into the water and prevent spread of disease or invasive species.
2. Collect subsamples from multiple locations around the waterbody/area you are sampling, using either the sampling bag provided to scoop up a small amount of water, or using a sterile ladle or empty mineral water bottle to transfer water into the bag.
For each sample, taking subsamples is an important part of the water collection process and typically increases detection rates. Ladles are a useful tool where the shoreline is difficult to access, and also allow equal amounts of water to be collected from each subsampling point. Sterile ladles can be purchased from NatureMetrics (contact us). In the absence of ladles, new mineral water bottles, with the water emptied, can be used in the same way.
3. Once a suitable amount of water has been collected (we recommend 3L for freshwater environments and 5L for marine environments), roll down the top of the sampling bag and shake for 20-30 seconds to thoroughly mix the subsamples.
4. Rest the bag in a sturdy place, either in a container to prevent the bag from tipping over, or with support from another person.
PART 2: FILTERING THE WATER AND PRESERVING THE DNA
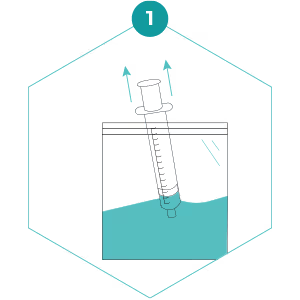
1. Draw up 50 mL of water from the sampling bag into the large syringe. If a Kemmerer/Niskin sampler was used to collect the sample, fill the sampling bag with water from the tap then draw up 50 mL into the large syringe. If there is a high sediment load, then leave the sample for a minute to allow the sediment to settle before drawing water up into the syringe. This should increase the amount of water that you can filter.
2. Attach the syringe to the inlet (narrow end). The syringe should easily twist onto the inlet side of the filter. Excessive twisting could damage the thread of the inlet and/or syringe tip. Stop twisting when you feel the slightest resistance – it is likely that only one or two twists will be required. Press the plunger to push the water through the filter.
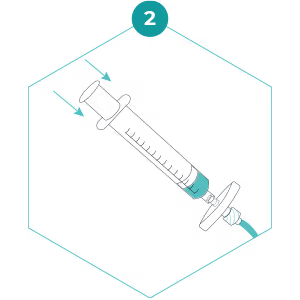
3. Repeat steps 1 and 2 (holding the filter in one hand while it is not attached to the syringe) until all water has been filtered or the filter clogs. Pass as much water through the filter as possible (see Advice Note for recommended volumes in different environments). Keep track of the number of times the syringe is refilled. Make a note on the sampling datasheet of the total volume processed.
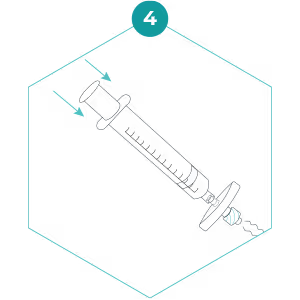
4. Detach the syringe from the filter, hold the filter in one hand, and pull back the plunger to fill the syringe with air. Reattach the filter and push the air through to expel any water trapped inside the filter. Repeat several times to remove as much water as possible. It helps to gently shake the filter as you do this.
5. The preservation buffer may precipitate (i.e. become cloudy) when kits are stored or used in cold temperatures. While precipitation will not adversely affect the preserving action, it is recommended to warm the small syringe in gloved hands for 1-2 minutes and then to gently shake to ensure proper mixing. Uncap the small syringe (already filled with preservation buffer) and twist it onto the filter inlet. Do not discard the Luer Lock cap – hold the cap in one hand as it will be needed in step 6. The solution is non-hazardous to aquatic life but release into the environment should be avoided. We advise performing this step away from the water’s edge and avoiding spills.
6. Hold the filter so that the outlet (wide end) points upwards. We advise you to do this step away from anyone’s eyes. Carefully and slowly press the plunger to push the preservation buffer into the filter. This must be done slowly to allow the preservation buffer to spread out over the filter surface. Stop when the first drop can be seen emerging from the filter outlet, but do not remove the small syringe with preservation buffer. Cap the filter outlet using the Luer Lock cap that was on the small syringe in step 5.
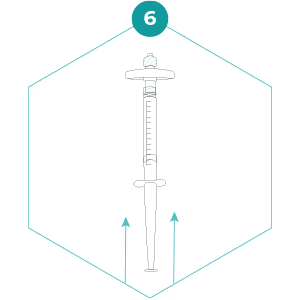
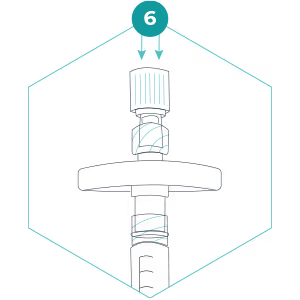
7. Invert the filter so that the filter outlet points down, and slowly press the plunger to expel the rest of the preservation buffer. The entire volume of preservation buffer should be added to the filter and the small syringe should be empty. Detach the small syringe whilst keeping the plunger depressed and cap the filter inlet with the separate Luer Lock cap. The preservation buffer contains a detergent – don’t be alarmed if it foams a little!
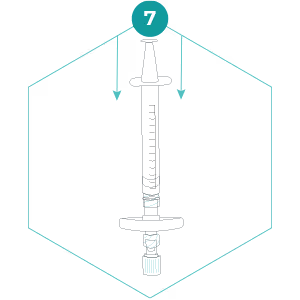
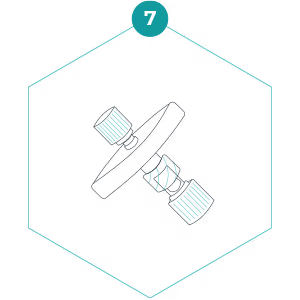
8. Place the filter inside the specimen bag (do not seal yet), then place the specimen bag inside the bag that the kit contents arrived in.
9. Repeat steps 1 – 8 for the remaining water, second filter, and second small syringe. If you have been able to pass more than half of the collected water through the first filter, then discard any remaining water and collect a second water sample in the same manner using the same sampling bag. Label the filters as ‘A’ and ‘B’ and record the volume of water filtered through each filter on the sampling datasheet.
10. Seal the specimen bag. If storing samples at ambient temperatures, samples should be received by the lab within 2 weeks of sampling. If this is not possible, we recommend that samples are frozen as soon as possible after sampling to enhance DNA preservation until samples are sent back to us. Cold shipping is not necessary for frozen samples. It should be noted that although freezing is recommended as best practice for optimal storage, we understand that it is not always feasible. Storing at ambient temperatures is a tried and tested method that yields excellent data. The addition of freezing is to further safeguard the samples against potential DNA degradation, which can affect species detection when samples are stored at high temperatures for long periods. Please contact NatureMetrics if return within 2 weeks or long-term freezing is not possible.
PART 3: RETURNING YOUR SAMPLES
Once you have collected and filtered your sample, follow our guidelines below to safely transport your sample to the NatureMetrics laboratory.
1. Complete the sampling datasheet using a pencil (do not use pen as ink can easily run, to the point where it becomes unreadable).
2. Place the bagged filter and sampling datasheet inside the bag that the kit contents arrived in.
3. Complete the sample manifest, which was attached to the email confirming shipping of your kits. Please record your sampling data on paper and on the sample manifest so there is a physical and electronic copy of your data.
4. For sample returns, visit our customer support desk here. Use the Logistics Request form to upload your completed sample manifest and book your sample return. NatureMetrics is fully compliant with current legislation on the transport of biological material and our operations team are on hand to ensure that all return shipments meet the required specification. We are only able to analyze samples that are returned using the correct NatureMetrics logistics procedure.
PACKAGING NOTES AND QUICK LINKS
- Return your samples using the box that kits were sent in, where possible.
- If using an alternative box, ensure that it is in good condition and remove any old labels.
- Avoid excess spacing by adding cushioning material (e.g. bubble wrap, newspaper) to avoid the product shifting inside the box.
- Use good quality sealing tape and seal along box edges in a H pattern.
- The only essential thing to send back to us is the specimen bag containing the filter and the sampling datasheet. However, all kit contents can be sent back to NatureMetrics for recycling.
CLIENT RESPONSIBILITIES
Important considerations for your sampling
- The absence of a detection does not necessarily imply the absence of a taxon from a location.
- Samples from some locations might have compounds that inhibit analyses resulting in reduced data generation – while we routinely test for inhibition, it is not always possible to overcome.
- It is the responsibility of the client to ensure that all efforts have been made to avoid contamination from external sources and between samples.
- Handling samples without gloves can increase the content of human DNA, reducing data generation.
- After sampling, movement of samples across borders without permission is not allowed as they are classed as biological samples.
Important considerations for interpreting your results
It should be noted that DNA can enter an ecosystem via many routes (e.g. wastewater from commercial and domestic sources). While DNA from a given taxon may be present and detectable, it is not possible to discern the source of the DNA. Results from common food items, domestic species and livestock species need to be interpreted with caution.
Disclaimer: Safe sample collection is the responsibility of the Client. NatureMetrics accepts no liability associated with the use of the kits and sample collection. The Client is solely responsible for the quality of the samples and the representativeness of the samples received by NatureMetrics. The information contained within the Final Report provided by NatureMetrics to the Client is not intended to be advisory, it is informational. Interpretation and decisions are the sole responsibility of the Client. NatureMetrics does not accept any liability whatsoever for any reliance placed on any information contained within, or any use that may be made of, the Final Report by the Client. Please read the full limitation of liability statement in the Terms and Conditions.
Health & Safety information for this kit can be viewed here.
See the following advice note for sampling tips on surveying ponds, lakes, rivers, streams, estuaries, seas, and oceans, as well as information on control samples and avoiding contamination.
